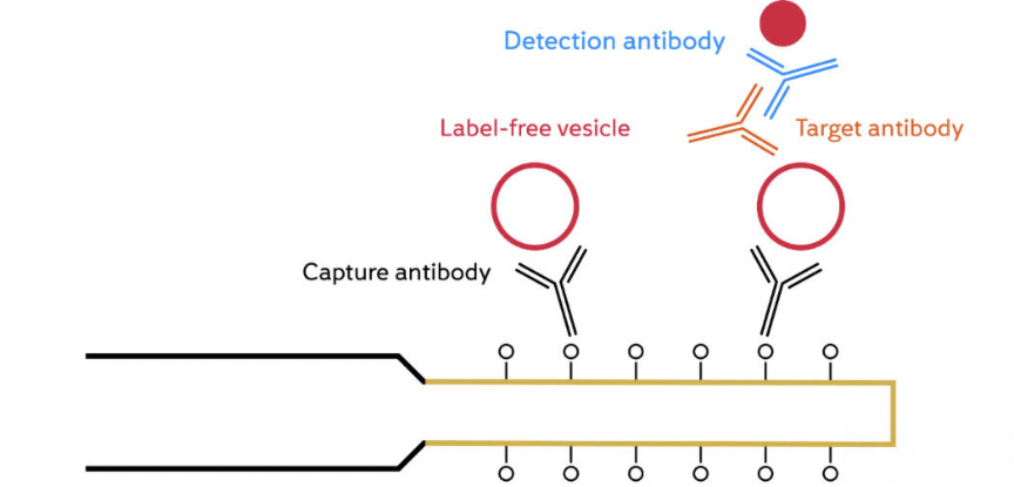
Extracellular vesicles (EVs) play a crucial role in mediating cell-to-cell communication. They are involved in several physiological and pathological processes within the body, including cancer development, and potential use as non-invasive cancer biomarkers. However, the extensive heterogeneity in size, composition, and origin of EVs makes their accurate and reliable characterization and detection crucial to meet […]
According to national statistics published by the government, between April 2020 and March 2021, 275,896 adults came into contact with drug and alcohol services (1). Testing for drugs of abuse can be used to a) discover drug abuse; b) monitor someone who has a substance abuse problem; c) detect drug intoxication and d) determine the […]
We are very proud of the academic work we undertake, and we have been assigned authorship on the following articles by our academic collaborators: C-Type Natriuretic Peptide (CNP) Inhibition of Interferon-γ-Mediated Gene Expression in Human Endothelial Cells In Vitro (click here to read article) Detection of equine atypical myopathy-associated hypoglycin A in plant […]
HPLC training in London Just in case you didn’t know – we offer this excellent value training. One to one by experts. If you have a project in mind, bring it along and work on it at the same time. Find out more
We were delighted to be asked to join the panel last night at the One Nucleus event hosted by LBIC at the RVC. The topic, ‘Staffing the 21st Century Life Sciences R&D Industry.’ As an employer of several graduates, undergraduates and summer placements in our 5 years of business, we were interested to be […]
Bio-Analysis Centre is reviewing and updating our privacy policies to ensure that your stored data is used securely and transparently. Please view our updated Privacy Policy here.
The Bio-Analysis Centre offers High Performance (label free) Capillary Electrophoresis (HPCE) system from deltaDOT to its clients. Whilst electrophoresis is the process during which ions undergo movement in a fluid or gel under the influence of an electric field, capillary electrophoresis is a technique that separates these ions based on their electrophoretic mobility with the […]
Chromatographic analysis is often an indispensable technique for a life scientist. The gadgetry and the skills required, for the successful application of such techniques, are less so common. Having spent the summer months of 2017, working for the Bio Analysis Centre, I have been a very keen observer of the company’s modus operandi. I have […]
As the global population continues to grow exponentially the demand for food becomes ever more pressing. In order to supply this mounting demand, the world’s crop production must increase through optimised methods, fertilisers, agrochemicals and pesticides. Nonetheless, with strict regulations regarding contaminants including pesticides, mycotoxins and heavy metals the manufacturing of food and drinks must […]
Analytical scientists utilise a range of sample preparation tools to aid their method development, how they approach this challenge can significantly impact their success. Solid Phase Extraction (SPE) is a type of sample preparation technology that uses solid particle and chromatographic packing material to chemically separate the different components of a sample. Samples are usually in […]