Detection of Breast Cancer‐Specific Extracellular Vesicles with Fiber‐Optic SPR Biosensor
Extracellular vesicles (EVs) play a crucial role in mediating cell-to-cell communication. They are involved in several physiological and pathological processes within the body, including cancer development, and potential use as non-invasive cancer biomarkers. However, the extensive heterogeneity in size, composition, and origin of EVs makes their accurate and reliable characterization and detection crucial to meet growing clinical demands. Although well-established conventional methods and emerging technologies are available, there is a lack of analytical instruments calibrated with reference material. Western blotting and enzyme-linked immunosorbent assay (ELISA) are the most favored conventional methods, and mass spectroscopy (MS) is a crucial analysis method. However, these methods have their limitations.
Emerging techniques, including magnetic detection, electrochemistry-based biosensors, and surface plasmon resonance (SPR), offer significant potential for EV analysis. However, the lack of a standardized material during the development of these approaches can seriously affect the reproducibility of results. Among various EV detection principles, SPR stands out with its capacity to enable real-time and label-free kinetics and affinity measurements.
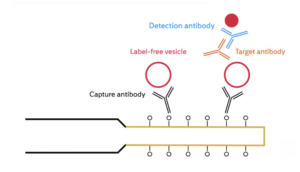
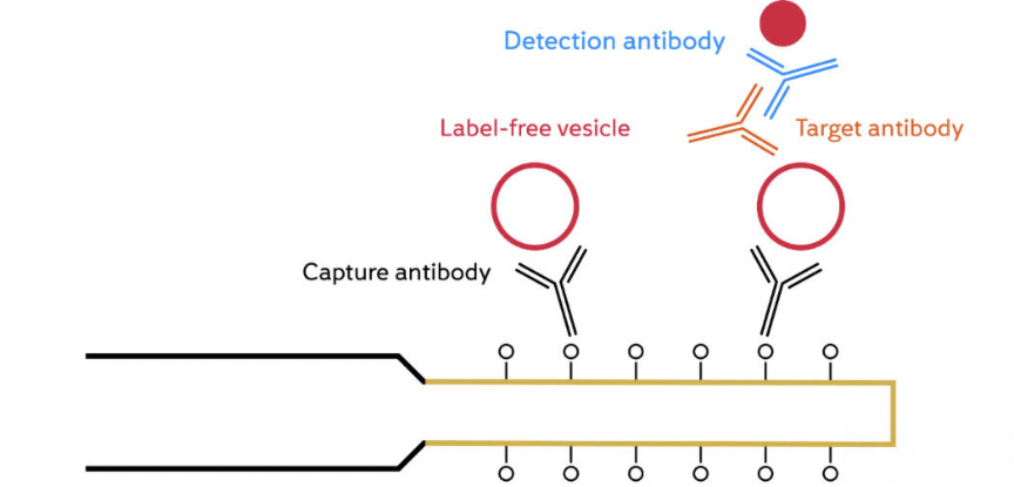
The development of reliable and standardised analytical instruments for EV detection and characterization is critical for the widespread use of EVs in cancer diagnostics, prognostics, and therapeutics. Researchers have developed a biosensor platform for detecting extracellular vesicles (EVs) from the SK-BR-3 breast cancer cell line. The platform is based on a fiber optic surface plasmon resonance (FO-SPR) sensor, which is functionalized with an antibody specific to the human epidermal growth factor receptor 2 (HER2), a protein that is overexpressed in SK-BR-3 cells.
The efficiency of antibody immobilization was optimized by screening buffers with different pH values and ionic strengths, and a concentration of 20 μg/mL was selected as the optimal antibody concentration. A sandwich bioassay was developed using biotinylated detection antibodies against tetraspanin proteins CD9, CD63, and CD81, which are commonly found on EVs. The combination of anti-HER2 and biotinylated anti-CD9 was selected as the preferred option for specifically detecting SK-BR-3 EVs. The calibration curves showed that the FO-SPR biosensor could detect SK-BR-3 EVs in buffer over the entire tested concentration range, with a limit of detection of 2.1 x 10^7 particles/mL. The biosensor was further tested in plasma and was found to be effective in detecting EVs.
The sandwich bioassay format allows for the colocalization of EV surface proteins, differentiating cancer-specific EVs from healthy cell EVs. It also enables real-time detection directly in crude samples, shortening the time without further optimisation to 2 hours and 40 minutes for cancer-specific EV detection, while avoiding lengthy isolation procedures.
Overall, it was found that the FO-SPR biosensor technology has the potential to be used as a standardized diagnostic tool and significantly contribute to the EV research field.
You can read more in this paper: https://doi.org/10.3390/ijms24043764