Liquid Chromatography Method Development
Why is Method Development important?
In the pharmaceutical sector, liquid chromatography procedures are used to assay compounds and quantify impurities present within medicinal products. Method development allows the creation of the best method to test for impurities and compounds which help ensure the quality, safety and efficacy of new products and drugs.
Liquid Chromatography Method Development
Method development is the process by which the optimal conditions are found for a particular compound to separate it from any contaminants. To design the best method for a particular sample, the following parameters and conditions must be optimized:
- Selection of chromatographic mode (Reverse Phase, Normal Phase,
Size exclusion, HILIC, etc.) - Selection of stationary phase (C18, C8, Phenyl-hexyl, Biphenyl,etc.)
- Selection of detector (UV, RID, MS, ELSD)
- Selection of mobile phase (which buffers; pH of buffers)
- Flow rate
- Preparation of samples (protein precipitation, Solid Phase Extraction,
etc) - Injection volume
- Concentration
- Calibration Range
HPLC Method Development
Our Nexera XR HPLC system (Fig.1) is ideal for developing procedures for a variety of material, ranging from complicated combinations of tiny molecules or proteins to single chemical purification.
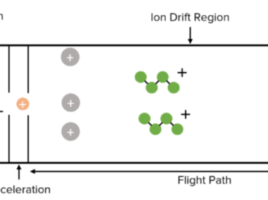
Mass Spectrometry Method Development
We have the expertise to design methodologies to quantify most drug-like substances using our Shimadzu LCMS-8040 (Fig.2).
Our manager Dr. Carolyn Hyde has over 20 years of experience developing methods on HPLC and LC-MS. Click here to contact us for more information on our services.
References
Avoomeen. Why Pharma Companies Must Invest in Method Development & Validation (Internet). (Cited on 16th July) Available from: https://www.avomeen.com/pharma-companies-invest-method-development/