Bioanalysis in Drug Discovery
Drug discovery/development covers different stages until a compound is proven to be effective and safe to use. A new drug’s route from original discovery to the marketplace takes at least ten years, with clinical trials alone requiring six to seven years on average. Each successful medicine is anticipated to cost $2.6 billion in research and development (2).
The evolution of bioanalysis as a vital instrument in the drug discovery and development process is well established and widely accepted. During the discovery stage, the purpose of bioanalysis might just be to produce suitable concentrations and/or exposure values that might be utilised to build a scientific basis for lead series identification and/or discrimination among various lead candidates (1).
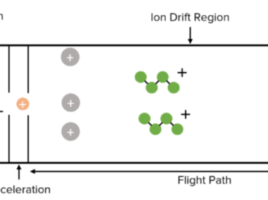
The term bioanalysis refers to the quantitative measurement of a molecule (drug) or its metabolite in biological fluids, primarily blood, plasma, serum, urine, or tissue extracts (1). First, in order to detect these compounds, samples must be prepared for analysis. For example, in most bioanalytical experiments, proteins are removed from the sample through either protein precipitation, liquid–liquid extraction, and solid phase extraction (SPE) (1). Second, a separation process is required, either high-performance liquid chromatography (HPLC) or HPLC coupled with mass spectrometry (HPLC-MS/MS) employing either electrospray ionisation (ESI) or atmospheric pressure chemical ionisation (APCI) technique. These are currently the most widely utilised methodologies in quantitative bioanalysis (1). However, before a bioanalytical method can be applied for normal usage, it must first be validated to show that it is adequate for its intended purpose. To support all development research, a GLP (Good Laboratory Practices) approved bioanalytical method is required (1). Validation entails demonstrating that the method is adequate and reliable for the intended analytical applications (1).
At the Bio-Analysis Centre, we offer HPLC and Mass-Spectrometry services. Our lab manager, Dr. Carolyn Hyde, is specialised in developing methods on HPLC and LC-MS for new compounds.
We also offer HPLC and Mass-Spectrometry training courses. You can find more information here.
References
- Pandey S, Pandey P, Tiwari G, Tiwari R. Bioanalysis in drug discovery and development. Pharm Methods. 2010 Oct;1(1):14-24
- PHRMA. Biopharmaceutical Research & Development: The process behind new medicines. (Internet) (Cited on 1st March) Available from: http://phrma-docs.phrma.org/sites/default/files/pdf/rd_brochure_022307.pdf