Adeno-associated virus (AAV) vector manufacturing for gene therapy products: How does the LCMS and HPLC support good manufacturing practice?
20 min read.
The adeno-associated virus (AAV) was first discovered in 1965 as a contaminant of adenovirus preparations, subsequently AAV was found in human tissues. Pure scientific curiosity in understanding basic AAV biology is what led to realising the tremendous potential of AAV as a vector in human gene therapies. AAV vectors are now the leading platform for human gene therapies (Wang et al., 2019).
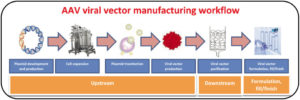
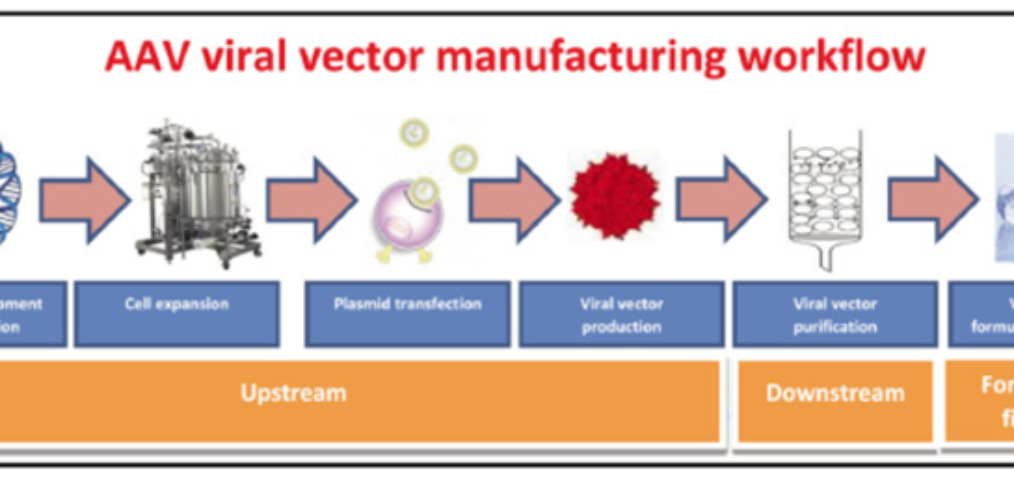
AAV manufacturing can be divided into three parts (as shown below) (Srivastava et al., 2021).
AAV manufacturing workflow – Upstream processing: AAV manufacturing starts with plasmid development. Then host cell numbers are expanded for plasmid transfection. These transfected cells produce the AAV. Downstream processing: Purification of AAVs occurs through filtration and chromatography methods. Formulation, fill/finish: Then the gene therapy product can be formulated. Diagram taken from: (Srivastava et al., 2021)
In commercial settings, characterisation, and high purity of the AAV serotypes in vector products are important parts of good manufacturing practice (GMP). From January of 2020, the US FDA recommends that all human gene therapy products be well characterised and evaluated to ensure high quality, in terms of potency, identity, and purity (Lam et al., 2022). This blog post will present reasons how the LCMS and HPLC could possibly facilitate achieving high quality gene therapy products which uses the AVV.
With increasing AAV research for gene therapies, novel capsids are becoming more prevalent in preclinical and clinical trials. Therefore, AAV capsid characterisation should be used as a drug quality control by confirming the AAV capsid in the drug (Lam et al., 2022). Characterisation of AAV capsid serotype typically is done by SDS-PAGE (Su et al., 2020, Penaud-Budloo et al., 2019). However, its results are moderately accurate, and these methods are time-consuming. The biggest benefit of using the LCMS is its’ high sensitivity and high accuracy. Recently, a new LCMS method has been developed for AAV serotype characterisation which is faster, more accurate and sensitive compared to previous methods (Lam et al., 2022). This method also has quick sample preparation.
One of the measured attributes to ensure drug efficacy, is the empty-to-full capsid ratio (i.e., the packaging efficiency). Analytical ultracentrifugation (AUC) and transmission electron microscopy (TEM) are commonly used to measure this ratio in biopharmaceutical companies. However, AUC requires a large sample volume and TEM is time-consuming (Hayes and Dobnik, 2022, Nam et al., 2023). HPLC is now increasingly being investigated as an alternative, to determine which seperation method is best between anion-exchange, cation exchange, and size exclusion chromatography. The benefit of HPLC assays is that they are accurate and sensitive. A limitation is that HPLC method development is time intensive and requires qualified personnel, therefore, HPLC is often not feasible in early product development. However, once the HPLC method is developed, it has the biggest potential for ensuring drug quality for commercial products compared to other methods (Gagnon et al., 2021, Heldt et al., 2023).
Chromatographic techniques, such as LCMS and HPLC, are popular in most commercial settings because once the method has been developed and validated, it is scalable. This is due to the ability of hiring less experienced personnel to perform assays on automated systems. Companies which aim to provide a platform for gene therapy innovators and manufacturers to deliver these drugs to patients, have clearly understood the importance in having chromatographic expertise part of this pipeline. For example, Astrea Bioseparations, which is a UK based leader in affinity chromatography, was the first company acquired by Gamma Biosciences, who are developing a complete production pipeline for cell and gene therapies.
Links to companies mentioned
Gamma Biosciences – https://gammabiosciences.com/
Astrea Bioseparations – https://www.astreabioseparations.com/
References
GAGNON, P., GORICAR, B., MENCIN, N., ZVANUT, T., PELJHAN, S., LESKOVEC, M. & STRANCAR, A. 2021. Multiple-Monitor HPLC Assays for Rapid Process Development, In-Process Monitoring, and Validation of AAV Production and Purification. Pharmaceutics, 13.
HAYES, D. B. & DOBNIK, D. 2022. Commentary: Multiplex dPCR and SV-AUC are Promising Assays to Robustly Monitor the Critical Quality Attribute of AAV Drug Product Integrity. J Pharm Sci, 111, 2143-2148.
HELDT, C. L., AREO, O., JOSHI, P. U., MI, X., IVANOVA, Y. & BERRILL, A. 2023. Empty and Full AAV Capsid Charge and Hydrophobicity Differences Measured with Single-Particle AFM. Langmuir, 39, 5641-5648.
LAM, A. K., ZHANG, J., FRABUTT, D., MULCRONE, P. L., LI, L., ZENG, L., HERZOG, R. W. & XIAO, W. 2022. Fast and high-throughput LC-MS characterization, and peptide mapping of engineered AAV capsids using LC-MS/MS. Mol Ther Methods Clin Dev, 27, 185-194.
NAM, Y. R., JU, H. H., LEE, J., LEE, D., KIM, Y., LEE, S. J., KIM, H. K., JANG, J. H. & LEE, H. 2023. Distinguishing between DNA-Loaded Full and Empty Capsids of Adeno-Associated Virus with Atomic Force Microscopy Imaging. Langmuir, 39, 6740-6747.
PENAUD-BUDLOO, M., BROUCQUE, F., HARROUET, K., BOUZELHA, M., SALEUN, S., DOUTHE, S., D’COSTA, S., OGRAM, S., ADJALI, O., BLOUIN, V., LOCK, M., SNYDER, R. O. & AYUSO, E. 2019. Stability of the adeno-associated virus 8 reference standard material. Gene Ther, 26, 211-215.
SRIVASTAVA, A., MALLELA, K. M. G., DEORKAR, N. & BROPHY, G. 2021. Manufacturing Challenges and Rational Formulation Development for AAV Viral Vectors. J Pharm Sci, 110, 2609-2624.
SU, Q., SENA-ESTEVES, M. & GAO, G. 2020. Analysis of Recombinant Adeno-Associated Virus (rAAV) Purity Using Silver-Stained SDS-PAGE. Cold Spring Harb Protoc, 2020, 095679.
WANG, D., TAI, P. W. L. & GAO, G. 2019. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov, 18, 358-378.